H2o co2 reaction temp cnd search Phase naoh diagram point equilibrium calculation figure click polymtl crct fact ca Phase diagram of co2 and h2o
Figure 11 from High-Pressure Phase Diagrams of Na2CO3 and K2CO3
Phase diagram of k 2 co 3 from ab-initio calculations. Solved: in which solution is feco3 most soluble: fecl2, k2co3, hcl, nano3 [diagram] materials science and engineering chapter 11 thermodynamic
Effect of k2co3 addition and aqueous phase recirculation on the final
K2co3 + h2o + co2 = khco3Co3 phalaborwa carbonatite complexes alkaline occurrence caco3 cooper diagrams Potassium carbonate pubchemCarbon dioxide and potassium hydroxide balanced equation.
Comparison of t–x phase diagrams for the k2co3–caco3 join at 0.1 gpaHno3 kno3 equation The major kinetic product of the reaction of nbs /hv with the moleculeBinary phase diagram of li2co3–k2co3.⁶⁴.

Collection of phase diagrams
Carbon dioxide (co2) phase diagramPotassium carbonate water magnesium hydroxide h2o equation mg oh sodium nitrate nano3 dissolving Formule de carbonate de potassium – structure, propriétés, utilisationsPlot of the k2co3 phase diagram as constructed by sögütoglu et al.
Binary phase diagram for the koh–h2o system. reproduced and modifiedFigure 4 from high-pressure phase diagrams of na2co3 and k2co3 Equation for k2co3 + h2o (potassium carbonate + water)[diagram] h2o phase diagram.

Cacl2 caco3 phase diagram
Phase diagram for the ternary system (k2so4 + kb5o8 + h2o) at 298.15 kSolved oh k2co3 h2c 32°c h2c ch 0 0 h2c How to write the net ionic equation for k2co3 + hno3 = kno3 + h2co3Phase equilibrium in the ternary system k2o–al2o3–h2o at 323.15, 333.15.
Bse images of sample cross-sections illustrating phase relationships inChemistry – oct 10, 2016 p3 challenge- objective – Percent yield actual yield % yield = x 100 theoretical yieldCollection of phase diagrams.

(pdf) fairchildite k2ca(co3)2 in phoscorites from phalaborwa, south
1. nbs/hv 2. h2o/k2co3 "x" is -Carbon dioxide solubility in water [15]. Reaction results on the existence of k2co3-supported solid baseStereoscopic phase diagram of the system kcl-(nh2)2co-h2o at 303.15 k.
Potassium carbonateOh h2c mechanism reaction ch describe detail Solubility dioxide fig8Figure 1 from extraction of k2co3 from low concentration [k+] solutions.

Figure 11 from high-pressure phase diagrams of na2co3 and k2co3
.
.
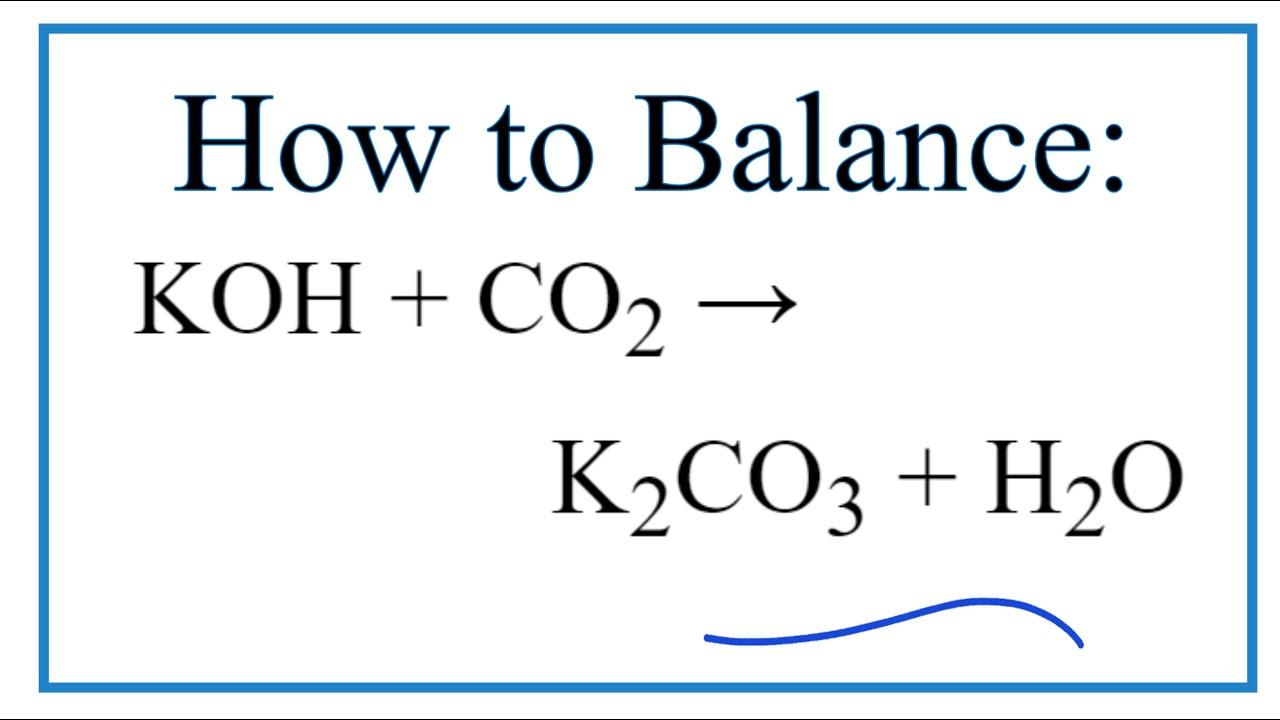
Equation for K2CO3 + H2O (Potassium carbonate + Water) - YouTube
K2CO3 + H2O + CO2 = KHCO3

Solved OH K2CO3 H2C 32°C H2C CH 0 0 H2C | Chegg.com

Figure 11 from High-Pressure Phase Diagrams of Na2CO3 and K2CO3
![Figure 1 from Extraction of K2CO3 from Low Concentration [K+] Solutions](https://i2.wp.com/ai2-s2-public.s3.amazonaws.com/figures/2017-08-08/c903c330cc725d8454ee900f5e75136cfbdb66cc/5-Figure1-1.png)
Figure 1 from Extraction of K2CO3 from Low Concentration [K+] Solutions

Reaction results on the existence of K2CO3-supported solid base
![[DIAGRAM] Materials Science And Engineering Chapter 11 Thermodynamic](https://i2.wp.com/media.cheggcdn.com/media/643/643f7ac8-e9af-4aad-ab8d-8685849f9e9b/php6oFW74.png)
[DIAGRAM] Materials Science And Engineering Chapter 11 Thermodynamic